

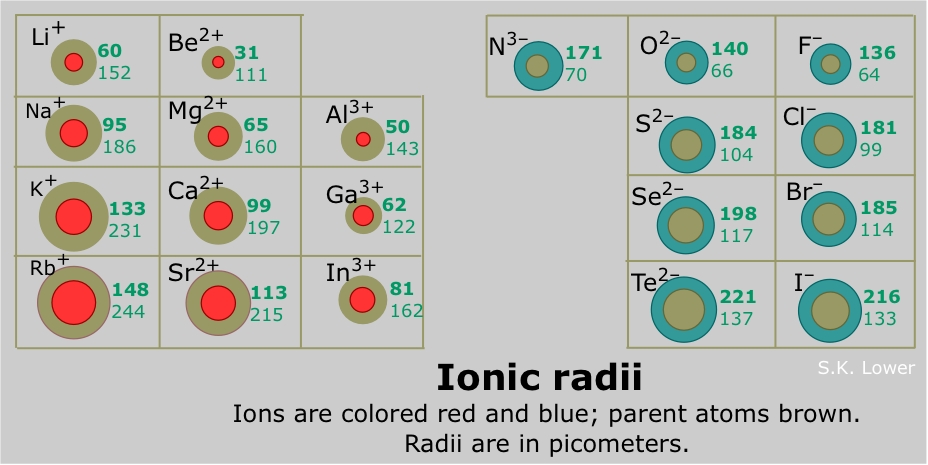
Therefore, it shows more attraction to its electrons. This is because helium has two protons on its nucleus. Note: We must know that out of all the elements present and known so far, Helium ($He$) has the smallest atomic radius. The electron-electron repulsion in Li+ ion is higher than in Li atoms. While the Earths atmosphere near the mean sea level contains 1019 atoms in a. Which of these elements has the smallest atomic radius Explanation: Helium has the smallest atomic radius. Thus, helium is the smallest element, and francium is the largest. Thus, from the given options, we can conclude that Bromine (\ ) has the smallest atomic radius as it belongs to halogens and so have covalent radius. The smallest ones are A-class, followed by B, C, M and X. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Helium has two protons so shouldn’t it be double the size no that’s wrong. Everyone thinks hydrogen is the smallest atom in terms of size in the whole periodic table because it has only one proton. You always remember that metallic radius is always higher than that of the covalent radius. Which atom is the smallest in the periodic table The most common wrong answer is hydrogen. Moreover, the radius of alkali metals is metallic radius, whereas the radius of halogens is covalent radius.
Or we can say that there is a stronger force of attraction that pulls the electrons closer to the nucleus which gives a smaller atomic radius. > Which one of the following has the small. > Classification of Elements and Periodicity in Properties. The concentration of more protons in the nucleus ultimately generates a higher effective nuclear charge. Which one of the following has the smallest atomic radius Class 11. Atomic radius decreases as we go across a period from left to right whereas the trend of atomic radii is seen decreasing as we move up a group from bottom to top.Įlectrons are added to the same energy level, as we move across a period and at the same time, protons are being added to the nucleus. This is due to trends in the periodic table.

Of the following, which atom has the smallest atomic radius Sr Ba Te. Variations in atomic radii are observed due to the effective nuclear charge that holds the valence electrons close to the nucleus. Solved Of the following, which atom has the smallest atomic . Explain why : ( i ) Chlorine atom ( atomic number 17 ) is smaller than sodium atom ( atomic. Group 1A of the periodic table are the alkali metals and from the given options sodium (\), potassium (\) and Lithium (\) belongs to alkali earth metals, whereas bromine (\) belongs to halogen family. And this is because all atoms add electrons to the same shell within the periods. Hint: We must know that the atomic radius decreases gradually from left to right across the period in the periodic table.


 0 kommentar(er)
0 kommentar(er)
